Overview
Sample cleanliness is extremely important for achieving good results in nanofabrication (hence the need for a cleanroom). However, simply working in the cleanroom does not guarantee cleanliness, proper steps need to be taken at every processing step to ensure a clean result. While some processes have more stringent cleanliness needs than others, some general rules apply for all types of lithography:
- Handle substrates/wafers with cleaned tweezers, not hands.
- Substrates should not be left exposed. Although the cleanroom offers nearly particle free air, particles are generated by the movement, plastic wafer carrier, talking, etc. Even in the cleanest areas of the cleanroom, particles will eventually find their way onto exposed surfaces.
Solvent Cleaning
Standard Process: TAI clean
- 5 min sonication in beaker of Toluene.
- 5 min sonication in beaker of Acetone.
- 5 min sonication in beaker of Isopropanol.
Background
Standard solvent cleaning is a three step process of ultrasonically agitating the substrate in subsequent solvent solutions to remove organic contamination. Three steps allow for selecting solvents that properly dissolve multiple types of contaminants. The first step is a nonpolar solvent (toluene) to remove any oils or grease present on the sample. The second step is a powerful polar solvent (acetone) to remove polar contaminants such as photoresist. The final step is a polar protic solvent (isopropanol).
Nonpolar Solvent Clean: Toluene
Toluene acts a nonpolar solvent to dissolve any grease or oil on the substrate. Nonpolar contaminants (especially fingerprints) will not be effectively removed by Acetone or IPA. (Interesting Fact: Toluene is used in the process to remove cocaine from coca leaves in the production of Coca-Cola syrup!!)
Polar Aprotic Solvent Clean: Acetone
Acetone's high dipole moment leads to a powerful cleaning of polar residue (such as photoresist). However its quick evaporation leads to residue redepositing on the substrate if evaporation is allowed to occur.
Polar Protic Solvent Clean: Isopropanol
IPA is used to remove fully wash away acetone from the substrate, and remove any remaining dissolved residue. As a result, IPA is mainly used to removing the Acetone without residue. However, unlike Acetone, IPA will form hydrogen bonds, and thus may provide some additional cleaning effects.
IPA is prefered as a final step over methanol. Its significantly slower evaporation rate results in less residue and a more forgiving rinse step. The close dipole moments of the two chemicals (DIPA=1.66 vs. DMethanol=1.69) means the difference between the two in cleaning ability is small, although methanol is somewhat stronger. From a safety standpoint, unlike Methanol, IPA is not considered toxic. And importantly for cleaning, "IPA is generally the purest available organic solvent and is used extensively for vapor drying of H2O-rinsed wafers." (Reinhardt 2008, p. 22)
If the clean is done immediately before spinning photoresist, a thorough IPA clean and dry should dehydrate the wafer, removing the need for a dehydration bake. If there is a significant delay between cleaning and spinning, ambient water will be adsorbed on the wafer surface. A dehydration bake should then be done.
Deionized Water Rinse
A final DI rinse is usually optional, but is required for samples to be placed in furnaces.
A powerful final step may be a rinse in the nanopure deionized water, which will assist in the removal of any remaining particles, solvent residues, and acid/base residues. However, if this step is employed before spinning photoresist, a dehydration bake must be performed to properly remove bound surface water molecules. This step is required for samples to be placed in any high temperature equipment or furnaces, as even slight solvent residue will combust at temperatures above 400 C.
Piranha Clean
Standard Process: Piranha Clean
- A completely dry glass container should be used.
- Add 3 parts Sulfuric Acid (H2SO4).
- Add 1 part Hydrogen Peroxide (H2O2).
- Allow the solution to work for 5-10 minutes.
- With sink running and while flushing with large amount of water, slowly pour used Piranha solution down drain.
Video SOP
Background
Piranha is a strong oxidizing agent that will strip surfaces of organic (carbon containing) matter and hydroxylate surfaces (add OH groups, making the surface hydrophilic). Note that Piranha will not remove inorganic surface contamination such as metals (Reinhardt 2008, p. 24) or particles. Piranha is self heating, and mixtures are typically used at the self heating temperature for 10 minutes. A more complete clean can be had from heating the mixture on a hotplate to 100-130 C for 10-15 minutes (Reinhardt 2008, p. 23).
- Samples should be free of large areas of organics. Piranha should not be used for removing a layer of PR off of a sample, a solvent clean should be used for large amounts of organics. Piranha is appropriate for cleaning off any remaining organic residue after a solvent clean, or for cleaning a new wafer. Attempting to strip large amounts of PR may result in a hardening of the PR layer (presumably due to the formation of undissolved elemental carbon), which will then be extremely difficult to remove.
- Piranha may roughen your substrate. Piranha is generally safe for use with inorganic substrates, but may roughen the surface on the nanoscale.
- Ratios of H2SO4 and H2O2 are important. Getting close to the 3:1 ratio (3 parts H2SO4: 1 part H2O2) is important. Ratios down to 7:1 will still give powerful cleaning, but something within the range of 3:1 to 7:1 should be used. Adding more peroxide (e.g. a 1:1 mixture) both presents a safety hazard and weakens the strength of the solution, and should not be done.
- Contrary to the conventional rule of "Always Add Acid" in chemistry, Piranha should always be prepared with acid first, and peroxide slowly added second. Improperly prepared Piranha may bubble violently when adding the chemicals, this is avoided by adding chemicals slowly and adding the sulfuric acid first.
- Water should NOT be added to Piranha solution and ALL beakers, tweezers and samples added to Piranha must be completely dry. Piranha is self heating, and can easily reach temperatures above 100 C. The addition of water can cause violent boiling.
- Piranha solution should be left uncovered, and under no circumstances should it be sealed. The oxygen and oxidation byproducts produced by the reaction are flammable, and sealing the container can cause them to explode.
- Piranha etches standard metal acid tweezers and will react explosively with "plastic" tweezers.
- Standard metal tweezers may be used safely with Piranha solutions, but the tweezers will be etched, and this etched iron will/may contaminate glassware and your sample.
- Carbofib tipped tweezers should not be used with Piranha. Carbofib is PEEK reinforced with carbon fiber, and will not be inert to Piranha, or other strong acids.
- "Plastic" tweezers should absolutely not be used with Piranha (or any metal).
- Teflon coated tweezers (typically green) should not be used, as Piranha will remove the outer coating and expose the bare metal underneath.
- Solid Teflon tweezers (PTFE) will be both chemically resistant to Piranha and will not present any contamination issues, but these are hard to hold samples with.
Piranha alternative: Nanostrip
Birck supplies Nanostrip, a stabilized Piranha alternative. Standard Nanostrip consists of 90% sulfuric acid (H2SO4), 5% peroxymonosulfuric acid (H2SO5), 5% water (H2O), and <1% hydrogen peroxide (H2O2). A more powerful version, Nanostrip 2X, consists of 85% sulfuric acid (H2SO4), 10% peroxymonosulfuric acid (H2SO5), 5% water (H2O), and <1% hydrogen peroxide (H2O2).
Nanostrip can be used at room temperature, but to be an effective cleaner, it should be heated to 60-80 C. More information can be found here: https://web.archive.org/web/20161023223906/http://cyantek.com/nano-strip.php
Room temperature Nanostrip is discouraged compared to either heated Nanostrip (to 60-80 C) or a freshly mixed Piranha solution due to:
- Decreased cleaning power.
- Difficulty of cleaning and contamination concerns. Room temperature Nanostrip is highly viscous (being 90% concentrated sulfuric acid), and is far more difficult to wash off than either heated Nanostrip or freshly mixed Piranha. While any "type" of Piranha presents potential sulfur contamination issues, room temperature Nanostrip is especially prone to contamination due to the difficulty in washing. Nanostrip residue may be left, which will not only contaminate your sample, but may also outgas in high temperature or vacuum systems, eating away the tool.
Oxide Growth and Removal
On silicon, Piranha will grow a contaminated oxide layer. This should be removed with a quick dip in HF/BOE, followed by thorough rinsing. As always with HF/BOE, all appropriate safety procedures and precautions MUST be followed.
Characterizing Cleanliness
Particle inspection (Particle size ~0.5 um and larger)
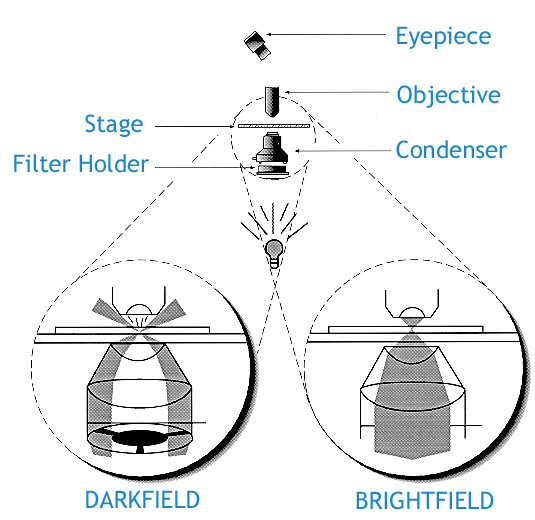
Inspection for contamination by particles 0.5 um or larger can be achieved readily through dark field microscopy.
In bright field illumination, the object is lit from below the stage, resulting in a larger, contrasted image that can be studied.
A dark field microscope is ideal for viewing objects that are unstained, transparent and absorb little or no light. These specimens often have similar refractive indices as their surroundings, making them hard to distinguish with other illumination techniques. A dark field microscope blocks this central light with a condenser so that only oblique rays hit the object. If there is nothing on the stage, the aperture of the condenser is greater than the objective and the view will be completely black. It is more useful in examining external details, such as outlines, edges, grain boundaries and surface defects than internal structure.
https://www.microscopeworld.com/t-darkfield_microscopy.aspx
Dark-field microscope (a) and top-view scanning electron microscope (SEM) (b) images of the same area on a silicon wafer ablated by a femtosecond laser. Microscope image is inverted in horizontal direction relative to that of the SEM. Selected nanoparticles are marked by corresponding numbers 1 to 6 in both figures.
Questions & Troubleshooting
Process Library
References
Presentations and Technical Sheets:
Solvents: Theory and Application (via Microchem)
Substrate Cleaning and Adhesion Promotion (Microchem)
Cleanliness, Contamination, and Chemical Handling (via SNF)
Textbooks:
Handbook of Silicon Wafer Cleaning Technology, Karen A. Reinhardt and Werner Kern, 2008.
Handbook of Semiconductor Manufacturing Technology, Robert Doering and Yoshio Nishi, 2007.
Piranha:
University of Cambridge Piranha guidelines
RCA Standard Clean: